Genetics
Understanding the Genetics of Congenital Sucrase-Isomaltase Deficiency
Congenital Sucrase-Isomaltase Deficiency (CSID) is an autosomal recessive disorder affecting the sucrase-isomaltase gene (SI). The sucrase-isomaltase (SI) gene is located on chromosome 31 and although SI constitutes one gene, it can be subdivided into two regions. One region of the gene encodes for the enzyme sucrase and the other region encodes for isomaltase.
In This Section
The SI gene is located on the long arm (q) of chromosome 3 at position 26.1.
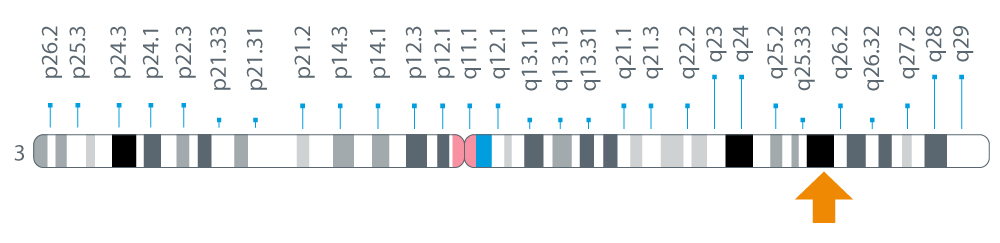
Figure 1: Location of the SI gene on chromosome 3 at position 26.11
There are many possible variants of the SI gene that code for the enzyme sucrase-isomaltase. Most of these variants are benign. However, in a number of individuals diagnosed with CSID, researchers have identified 37 gene variants that code for malfunctioning sucrase-isomaltase, the enzyme associated with CSID.2-10
In a recent study by Uhrich et al, the SI gene of 31 patients with documented CSID was sequenced to identify mutations and variants in the gene sequence.2 Mutations that manifest as CSID symptoms typically alter the structure, disrupt the production, or impair the transport, causing the enzyme to malfunction.
Among these 37 gene variants, 4 were the most common variants identified.2 Fifty-six mutated alleles were identified in this study, with 4 of the 56 mutations found in 32 of the mutant alleles.2 The 4 most common SI gene mutations or variants detected were: Gly1073Asp, Phe1745Cys, Val577Gly, and Arg1124Stop.2
The majority of the variants were found in the sucrase domain of the SI gene (58%) as compared with the isomaltase domain (43%), even though isomaltase accounts for a larger proportion of the gene sequence than sucrase.2 This may help to explain why most CSID patients have little to no sucrase activity but varying degrees of isomaltase activity when assayed by a disaccharidase enzyme activity test.
At least 1 of the 4 most common variants was present in 59% of the alleles of 31 patients examined, which represents an approximate heterozygote frequency of 83% of symptomatic children of European descent. By comparison, the estimated heterozygote frequency retrieved from a public database of theoretically normal controls, the Exome Aggregation Consortium (ExAC) database is 0.8%.2 These results suggest there could be more than two million in the United States who have a heterozygous variant associated with CSID. Further studies are underway to more accurately determine the prevalence of CSID.
There are significant variations in the symptoms experienced by patients with CSID. This is not surprising, considering the relatively large number of genetic mutations or variants that were discovered in the SI gene in a relatively small group of patients. While all the patients in the Uhrich study lacked sucrase activity, some had normal isomaltase activity, some had only traces of isomaltase activity, and others had reduced but sufficient isomaltase activity. These variations in symptom type and severity are known as phenotypic variations.2
These genetic mutations cause functional gastrointestinal problems such as diarrhea, recurrent abdominal pain, and bloating. Because these symptoms are common to many other gastrointestinal disorders, such as irritable bowel syndrome (IBS), some scientists believe that a proportion of patients who are diagnosed with other gastrointestinal disorders such as IBS may actually suffer from undiagnosed CSID or are heterozygotes for a pathogenic SI gene.
Further studies are underway and could help more accurately determine the prevalence of CSID in patients diagnosed with IBS and other gastrointestinal disorders associated with chronic diarrhea. Due to the relative rarity of the disorder, it is possible that a significant proportion of affected pediatric and adult patients are not being tested, and therefore not being diagnosed with CSID or adequately managed.
References
- MedlinePlus. SI gene. Last updated July 1, 2008. https://medlineplus.gov/genetics/gene/si/#references
- Uhrich S, Wu Z, Huang J, Scott CR. Four mutations in the SI gene are responsible for the majority of clinical symptoms of CSID. J Pediatr Gastroenterol Nutr. 2012; 55(2):S34-S35. doi:10.1097/01.mpg.0000421408.65257.b5
- Alfalah M, Keiser M, Leeb T, et al. Compound heterozygous mutations affect protein folding and function in patients with Congenital Sucrase-Isomaltase Deficiency. Gastroenterology. 2009;136(3):883-92. doi:10.1053/j.gastro.2008.11.038
- Gericke B, Amiri M, Naim HY. The multiple roles of sucrase-isomaltase in the intestinal physiology. Mol Cell Pediatr. 2016;3(1):2. doi: 10.1186/s40348-016-0033-y.
- Jacob R, Zimmer KP, Schmitz J, et al. Congenital Sucrase-Isomaltase Deficiency arising from cleavage and secretion of a mutant form of the enzyme. J Clin Invest. 2000;106(2):281-7. doi:10.1172/JCI9677
- Keiser M, Alfalah M, Pröpsting MJ, et al. Altered folding, turnover, and polarized sorting act in concert to define a novel pathomechanism of Congenital Sucrase-Isomaltase Deficiency. J Biol Chem. 2006;281(20):14393-9. doi:10.1074/jbc.M513631200
- Naim HY, Heine M, Zimmer KP. Congenital Sucrase-Isomaltase Deficiency: heterogeneity of inheritance, trafficking, and function of an intestinal enzyme complex. J Pediatr Gastroenterol Nutr. 2012;55(suppl 2):S13-20. doi: 10.1097/01.mpg.0000421402.57633.4b
- Ritz V, Alfalah M, Zimmer KP, et al. Congenital Sucrase-Isomaltase Deficiency because of an accumulation of the mutant enzyme in the endoplasmic reticulum. Gastroenterology. 2003;125(6):1678-85. doi:10.1053/j.gastro.2003.09.022
- Sander P, Alfalah M, Keiser M, et al. Novel mutations in the human sucrase-isomaltase gene (SI) that cause congenital carbohydrate malabsorption. Human Mutat. 2006;27(1):119. doi: 10.1002/humu.9392.
- Spodsberg N, Jacob R, Alfalah M, et al. Molecular basis of aberrant apical protein transport in an intestinal enzyme disorder. J Biol Chem. 2001;276(26):23506-10. doi:10.1074/jbc.C100219200